ERSU: A Cell Cycle Checkpoint for the ER in Mammalian and Yeast Cells
ERSU: A Cell Cycle Checkpoint for the ER in Mammalian and Yeast Cells
These features of the ER pose a major challenge when dividing the ER into two daughter cells. Specifically, the ER cannot be generated from scratch, but instead can only be inherited from a cell with an existing ER. In addition, division of the ER must coordinate with the cell cycle division of the nucleus. These features raise the intriguing question of whether a cell division checkpoint-like mechanism exists to ensure that the ER properly segregates from the mother to the daughter cell during division. Similarly, little is known about possible cycle checkpoints for properly dividing other cytoplasmic compartments.
Dissecting the ERSU checkpoint
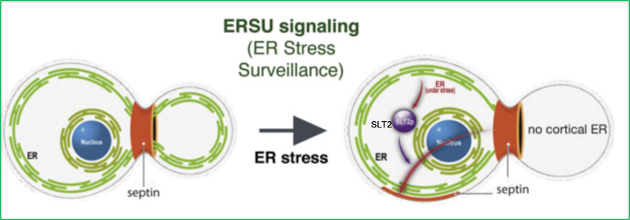
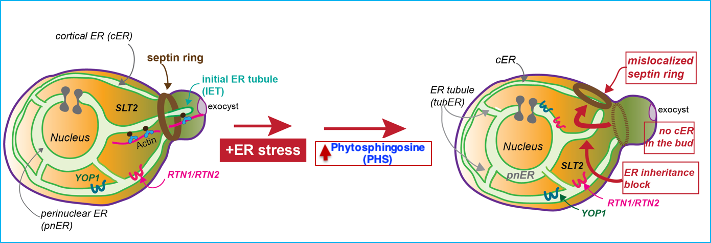
We discovered a cell cycle checkpoint that ensures that all dividing cells receive both functional and spatially sufficient ER during the cell cycle, which we termed the ER stress surveillance or ERSU cell cycle checkpoint. We discovered this novel checkpoint in the genetically tractable experimental system S. cerevisiae. The ERSU checkpoint is unique in that it monitors cytoplasmic organelle function. During the cell cycle, if the physical size or functional capacity of the ER is not sufficient to give rise to two dividing cells, ER inheritance and cell division (cytokinesis) are blocked (Babour et al., Cell 2010; reviewed in Niwa, 2020). Under ER stress conditions, preventing timely entry into the cell cycle holds the key to cell longevity, underscoring the functional importance of this checkpoint. Using advanced microscopy, yeast genetics, and molecular and cell biological approaches, we are investigating what triggers the ERSU, and dissecting the molecular mechanisms of this checkpoint in response to ER stress.
Additional Papers: Pina et al., 2015; Pina et al., 2016; Pina and Yagisawa et al., 2018; Chao et al., 2019
An emerging ERSU checkpoint in mammalian cells
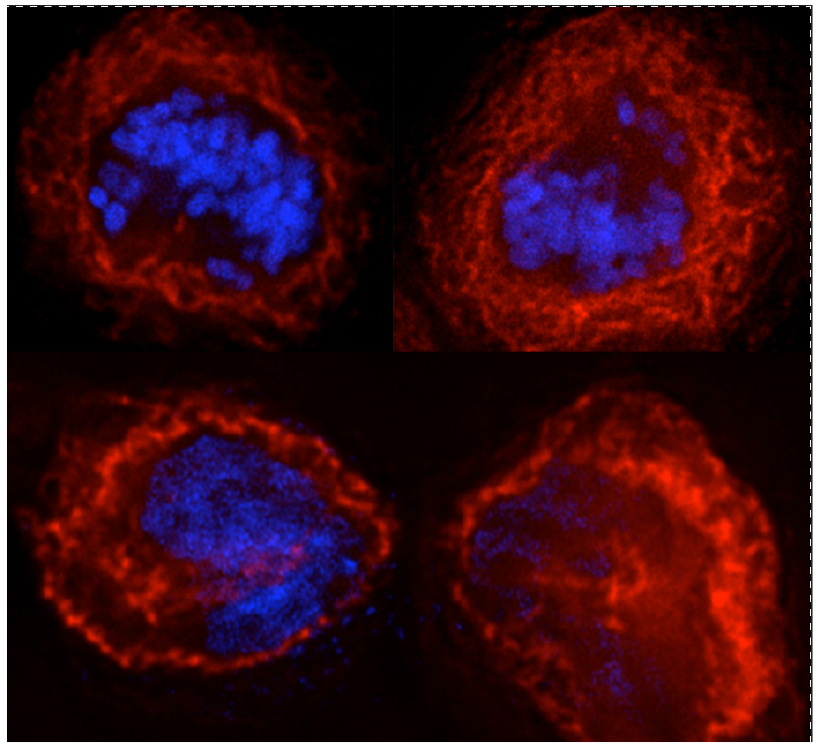
In addition to yeast, we are investigating a similar cell cycle checkpoint for ER division in mammalian cells. The choreographed mechanism of the mammalian cell cycle differs from that in yeast significantly. During mitosis, the mammalian nuclear envelope (NE) breaks down, allowing the mitotic chromosomes to initiate spindle formation in the cytoplasm. The nuclear membranes retract into the ER, where nuclear membrane proteins and lipids become mixed into the extensive web-like ER network. Following chromosome separation and before daughter nuclei can form, the ER must be cleared away from the chromosomes. Nuclear envelopes then reassemble around each set of daughter chromosomes — by retrieval of membranes, nuclear membrane proteins, and a subset of nuclear pore proteins from the ER. Ultimately, this leads to closure of the nuclear membranes, completion of nuclear pore formation, creating two dynamic mammalian nuclei.
Considering these dynamic choreographed changes, we hypothesize that ER functional homeostasis is even more tightly orchestrated with that of chromosome replication and separation. Indeed, our recent experimental results strongly suggest that functional disruption of the ER impacts the mammalian cell cycle and that an ERSU cell cycle checkpoint(s) exists in mammalian cells. Using state-of-the-art live cell imaging, CRISPR/Cas9-based and pharmacological high-throughput screening, and molecular and biochemical approaches, we are defining and investigating the ERSU cell cycle checkpoint for mammalian cells.
Furthermore, we anticipate that the ERSU checkpoint that we discovered in the budding yeast will provide molecular grounds to jump-start our investigations into how ER division occurs during stem cell differentiation, where cell division occurs asymmetrically.